背景
FMS系列能量代谢监测系统方案作为SSI家族一款经典、坚固耐用、多用途的高精度高分辨率代谢测量主机,受到全世界以各类昆虫、实验动物、小型及中大型野生动物、家禽家畜、人体等为研究对象的生理学、生态健康、生物医学科学家的极度青睐。FMS的再度升级改版,以更小体积、更大的数据储存容量、智能化大触摸屏、更简化的操作、更合理的价格将再次引爆专注于实验研究科学家灵活机动的创新性生物新陈代谢研究热情。

应用领域
野生动物(含媒介动物)适应环境的行为、生理、进化等研究
以实验动物为模型的肥胖、心血管、糖尿病、衰老等健康研究
以家畜家禽等经济动物为研究对象的营养学、温室气体排放等研究
以人体为研究对象的运动生理学、环境模拟生理学、特殊人群营养学等健康研究
技术特点
全新迷你型主机,坚固的外壳,带搬运手柄,具有便携性,可在各种复杂野外环境条件下现场使用
面板32GB SD卡数据存储允许即时存储信息,而无需单独的计算机
温度气压自动补偿,消除环境温度气压变化引起的误差
8通道模拟信号输入,可兼容其它分析仪或传感器,4通道温度输入
超大触摸屏实时显示仪器各参数,可同时显示氧气、二氧化碳、水汽压、大气压、相对湿度、模拟输入信号、储存大小、取样情况、日期时间序列等数据
具备功能强大的扩展端口,可以组成多通道或各种因素控制的全面新陈代谢监测系统
具备电源线或锂离子电池包(4.8 A-H),野外运行时间至少6小时

技术指标
1.传感器:O2分析仪,燃料电池技术,使用寿命约2年,燃料电池可更换;CO2分析仪,无色散双波长红外气体分析仪;水汽分析仪,薄膜电容传感器
2.测量范围:O2,0 -100;大气压,30-110 kPa;CO2,0 – 5%;水汽压,0-0 -100RH(无凝结),温度0-100°C
3.精度:O2:2-100读数的0.1%;CO2:0-5%读数的1%;H2O:0-95% RH读数的1%,95-100优于2%;温度0.2® C
4.分辨率:O2: 0.001%;CO2: 0.0001%-0.01%;H2O: 0.001%RH
5.信号漂移:温度恒定的情况下O2: <0.02%每小时;CO2: <0.001%每小时;H2O: < 0.01%RH每小时
6.信号输入:八个标准电压双极模拟输入,四个温度输入
7.模拟输出:O2, CO2, 2个自定义
8.数字控制输出:8个TTL逻辑信号
9.数字输出:USB 到RS-232,Sablebus快速接口
10.内置存储器:SD存储卡,可达32GB
11.存储时间间隔:0.1sec到1hr用户自定义
12.气流流量:10-1500mL/min
13.流量控制精度:读数的2%
14.流量分辨率:0-99.9mL/min为0.1mL/min;100mL/min 以上为1mL/min
15.工作温度:3-50 °C,无冷凝
16.供电:12-15 VDC,带220V交流电适配器;可选配锂电池供电,方便野外操作。
17.尺寸:35cm×30cm×15cm
18.重量:4kg
19.呼吸室和代谢测量方案定制(如下图)

典型应用一
Comparison of the CO2 ventilatory response through development in three rodent species: Effect of fossoriality,Sprenger R J, Kim A B, Dzal Y A, et al. Respiratory physiology & neurobiology, 2019, 264: 19-27.
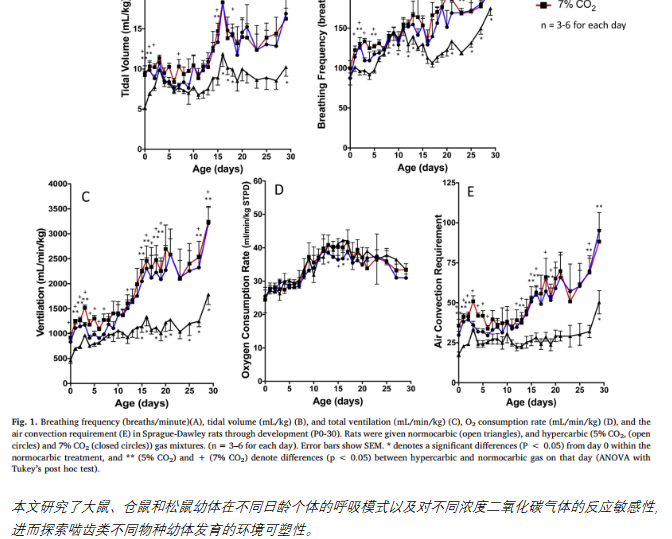
典型应用二
Greater energy demand of exercise during pregnancy does not impact mechanical efficiency,Denize K M, Akbari P, da Silva D F, et al. Applied Physiology, Nutrition, and Metabolism, 2019.
美国妇产科学院和加拿大的妇产科医生协会发表了的孕妇活动指南,建议孕妇进行150分钟中等强度运动以减少妊娠并发症,有利于母体和婴儿的健康。然而怀孕(婴儿作为特殊负重)是如何影响孕妇的能量投入、活动体能和机械效率的却了解很少。该研究通过FMS便携式能量代谢仪来定量化不同运动程序的能量消耗和机械效率。

产地
美国
部分参考文献
1.Charters J E, Heiniger J, Clemente C J, et al. Multidimensional analyses of physical performance reveal a size‐dependent trade‐off between suites of traits[J]. Functional Ecology, 2018, 32(6): 1541-1553.
2.Cochran J P, Haskins D L, Eady N A, et al. Coal combustion residues and their effects on trace element accumulation and health indices of eastern mud turtles (Kinosternon subrubrum)[J]. Environmental Pollution, 2018, 243: 346-353.
3.de Melo Costa C C, Maia A S C, Nascimento S T, et al. Thermal balance of Nellore cattle[J]. International journal of biometeorology, 2018, 62(5): 723-731.
4.Denize, Kathryn M., et al. "Greater energy demand of exercise during pregnancy does not impact mechanical efficiency." Applied Physiology, Nutrition, and Metabolism ja (2019).
5.Fernandes M H M R, Lima A R C, Almeida A K, et al. Fasting heat production of S aanen and A nglo N ubian goats measured using open‐circuit facemask respirometry[J]. Journal of animal physiology and animal nutrition, 2017, 101(1): 15-21.
6.Fonseca V C, Saraiva E P, Maia A S C, et al. Models to predict both sensible and latent heat transfer in the respiratory tract of Morada Nova sheep under semiarid tropical environment[J]. International journal of biometeorology, 2017, 61(5): 777-784.
7.Friesen C R, Johansson R, Olsson M. Morph‐specific metabolic rate and the timing of reproductive senescence in a color polymorphic dragon[J]. Journal of Experimental Zoology Part A: Ecological and Integrative Physiology, 2017, 327(7): 433-443.
8.Guigueno M F, Head J A, Letcher R J, et al. Early life exposure to triphenyl phosphate: Effects on thyroid function, growth, and resting metabolic rate of Japanese quail (Coturnix japonica) chicks[J]. Environmental pollution, 2019, 253: 899-908.
9.Haskins D L, Hamilton M T, Stacy N I, et al. Effects of selenium exposure on the hematology, innate immunity, and metabolic rate of yellow-bellied sliders (Trachemys scripta scripta)[J]. Ecotoxicology, 2017, 26(8): 1134-1146.
10.Ivy C M, York J M, Lague S L, et al. Validation of a pulse oximetry system for high-altitude waterfowl by examining the hypoxia responses of the Andean goose (Chloephaga melanoptera)[J]. Physiological and Biochemical Zoology, 2018, 91(3): 859-867.
11.Ladds M A, Slip D J, Harcourt R G. Swimming metabolic rates vary by sex and development stage, but not by species, in three species of Australian otariid seals[J]. Journal of Comparative Physiology B, 2017, 187(3): 503-516.
12.Lenard A, Gifford M E. Mechanisms Influencing Countergradient Variation in Prairie Lizards, Sceloporus consobrinus[J]. Journal of Herpetology, 2019, 53(3): 196-203.
13.Louppe V, Courant J, Videlier M, et al. Differences in standard metabolic rate at the range edge versus the center of an expanding invasive population of Xenopus laevis in the West of France[J]. Journal of Zoology, 2018, 305(3): 163-172.
14.Maia A S C, Nascimento S T, Carvalho M D, et al. Enteric methane emission of Jersey dairy cows: an investigation on circadian pattern[C]//21ST INTERNATIONAL CONGRESS OF BIOMETEOROLOGY. 2017: 100.
15.Nascimento C C N, de Fran®a Carvalho Fonsêca V, de Melo Costa C C, et al. Respiratory functions and adaptation: an investigation on farm animals bred in tropical environment[J]. 2017.
16.Noren D P, Holt M M, Dunkin R C, et al. Echolocation is cheap for some mammals: Dolphins conserve oxygen while producing high-intensity clicks[J]. Journal of experimental marine biology and ecology, 2017, 495: 103-109.
17.Otálora-Ardila A, Flores-Martínez J J, Welch K C. The effect of short-term food restriction on the metabolic cost of the acute phase response in the fish-eating Myotis (Myotis vivesi)[J]. Mammalian Biology, 2017, 82(1): 41-47.
18.Sanguino R A. Rapamycin Interacts with Nutrition to Decrease Basal MetabolicRate of Drosophila melanogaster[M]. Adelphi University, 2017.
19.Sprenger R J, Kim A B, Dzal Y A, et al. Comparison of the CO2 ventilatory response through development in three rodent species: Effect of fossoriality[J]. Respiratory physiology & neurobiology, 2019, 264: 19-27.
20.Toler M. Kinetics and Energetics of Feeding Behaviors in Daubentoniamadagascariensis[D]. Duke University, 2017.